New Certis Whitepaper: Bringing Certainty to PDX Model Characterization
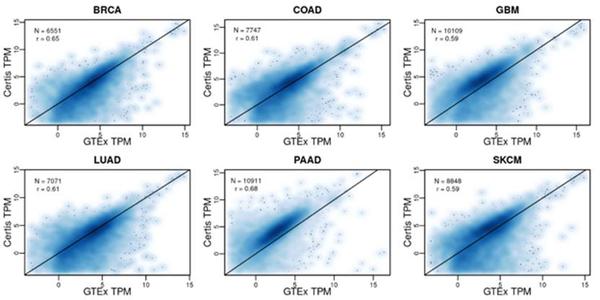
Patient-derived xenograft (PDX) models are indispensable for translational cancer research. However, public and private PDX tumor banks may lack standard quality controls, which may lead to genomic and transcriptomic characterization inaccuracies, and the potential to reduce efficiencies with which oncology researchers and clinicians can mimic tumor pharmacology, growth, metastasis, resistance and relapse. To demonstrate the quality of the Certis tumor bank and to ensure the data in the BarneyOI